Producing Imines and Enamines
Imines and enamines are good substitutes for enols. However, it is important to note how to convert the carbonyl compounds into these enamines and imines. The normal method of doing so is by just reacting the carbonyl compound with the amine to get the enamine.

For this reaction to work, however, the carbonyl compound must be activated to do this reaction. Alternatively, synthesis of imines and enamines may be done using silyl-amine type of compounds. This allows the reaction to be done in very mild conditions and giving good yields for sensitive carbonyl compounds as well as for those compounds which are less reactive.
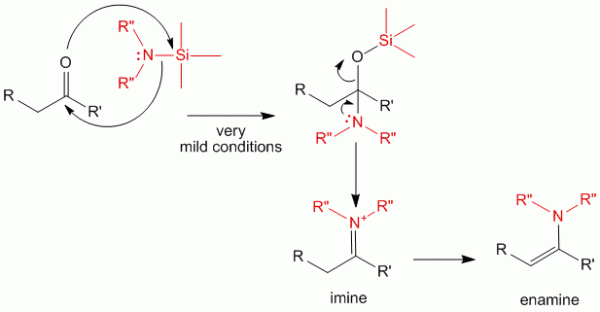
The mechanism above shows that the silyl group has a great affinity for the electronegative oxygen species allowing the first step of the reaction to proceed. This intermediate quickly undergoes a transformation to produce the imine and the enamine.
Using the Imines and Enamines
As mentioned before, imines and enamines are useful alternatives to enolates. Hence any reaction which can be done with enols and enolates can be done with the enamines, under much milder conditions. However the product will still have an enamine. To conver the enamine back to the carbonyl compound the product must be treated by acidic hydrolysis.

Enamines may also be used for created regioselectivity in reactions.
When cyclohexanone is reacted with pyrrolidine the product formed is an enamine. This enamine has planarity due to resonance as has been highlighted in the image with the bold lines.
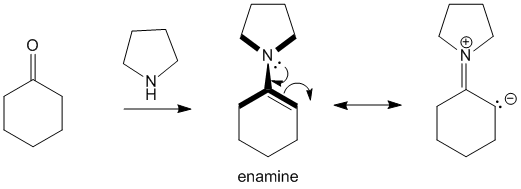
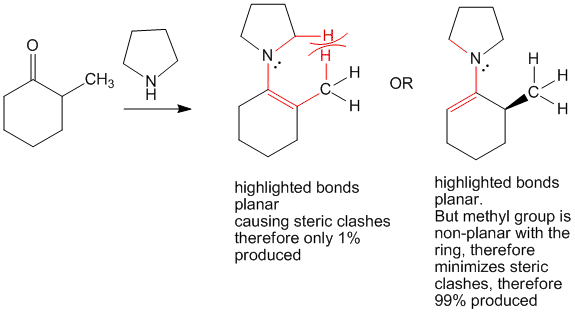
In case of 2-methyl cyclohexanone, this planarity would cause steric clashes between the methyl group and the pyrrolidine hydrogen atoms. Due to this, formation of the less stable 6-position carbanion is preferred and hence forces reactions to proceed at this position.
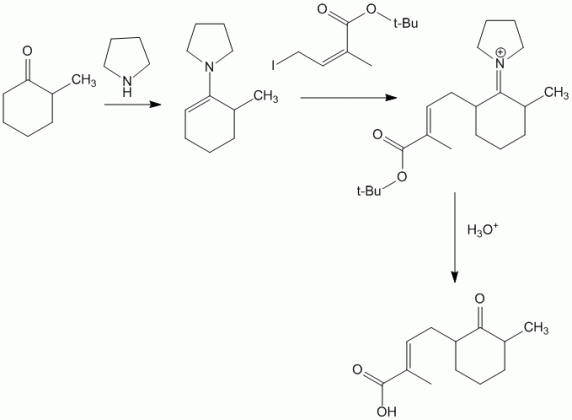
This technique is useful to perform regioselective reactions at less reactive centers. For example,
Books on Organic Chemistry
Check out some of the best textbooks for Organic Chemistry
References
- Advanced Organic Chemistry: Reactions and synthesis. By Francis A. Carey, Richard J. Sundberg
- Organic Chemistry, 6th Edition. By Robert T. Morrison and Robert N. Boyd