Enolates can be very tricky to handle. Upon reaction with a base and in the presence of alkyl halide, the carbonyl compounds with α-hydrogens can undergo a reaction to produce a mixture of c-alkylated product or o-alkylated product. This occurs due to the formation of enolate ion as will be seen ahead in the mechanism.

Mechanism
Step 1
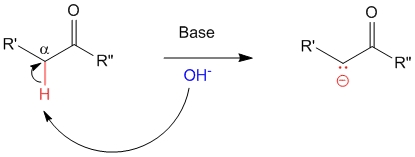
In the first step, the base abstracts the alpha-hydrogen of the carbonyl containing compound (in this case a ketone).
Step 2
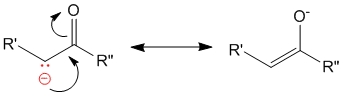
In the second step, the carbanion formed can undergo resonance stabilization to form the enolate ion.
Step 3
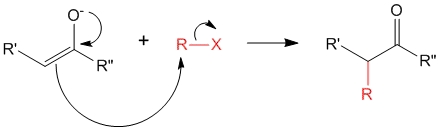
The enolate ion can now react with the alkyl halide (R-X) to produce either
1. C – Alkylation
2. O – Alkylation
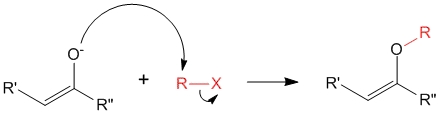
Factors determining C vs O alkylation
A number of factors such as negative charge density, solvation, cation coordination, strength of electrophile and product stability affect whether the reaction progresses to C – alkylation or to O – alkylation. Often, in most reactions, a mixture is obtained and the ratios vary from reaction to reaction.
For example3,
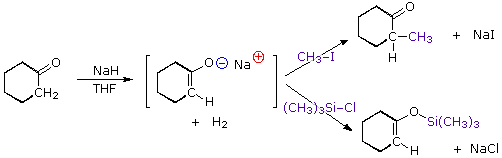
In case when this reaction occurs with methyl iodide – Methyl iodide is a weak electrophile. Also the cation coordination is strong with the O– as it has greater negative charge density. Due to these facts the C-alkylated product forms the more thermodynamically stable product.
In case when this reaction occurs with trimethyl silyl chloride – Trimethyl Silyl Chloride is a much stronger electrophile as compared to methyl iodide. The SN2 transition state will resemble the reactants (enolate) more than the products. Also the reaction at the more negative charge density which is the oxygen atom, will be more preferred. Also, since O-Si bond is very stable (25 Kcal more favorable than C-Si bond), the reaction would prefer to go via the o-alkylation thermodynamically as well as kinetically.
Books on Organic Chemistry
Check out some of the best textbooks for Organic Chemistry
References
- Advanced Organic Chemistry: Reactions and synthesis. By Francis A. Carey, Richard J. Sundberg
- Organic-Chemistry.org (accessed on February 25, 2011)
- http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/aldket2.htm (accessed on February 25, 2011)