Introduction
In this page we discuss a few examples of how we can utilize the Woodward-Fieser rules to determine the wavelength of maximum absorption for some molecules. We highly recommend that you read up the first two sections on the Woodward rules to calculate the λmax for conjugated dienes and the Woodward rules to calculate the λmax for unsaturated carbonyl compounds, before you read this page.
Note: Numerical values for Woodward-Fieser rules differ slightly from one textbook to another. We have tried to compile an extensive list of numerical values from online resources, textbooks and journal articles based on the popularity of the number. It is recommended that you learn on how to apply the values for the contributors and then follow the values given in a text book recommended by your teacher, or use our values. We believe that learning how to apply the rules is more essential than actually getting the exact answer. Other’s opinions may vary.
In these sample problems you will be shown the structure, then the structure is highlighted to show you key features which would affect the λmax of the molecule. Then the table will show you the solutions on how to solve to get the wavelength of maximum absorption, with a final calculated λmax using the Woodward-Fieser rules. In some cases if we have an observed λmax for comparison, it may be given as well.
Note- If you have your own problems please write the IUPAC name in the comments section and I will attempt to solve it and add it to this list of examples.
Example/Sample Problem 1
Example/Sample Problem 2
Example/Sample Problem 3
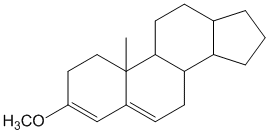
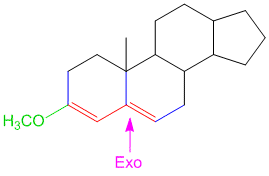
Example/Sample Problem 4
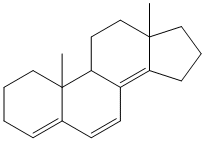
Example/Sample Problem 5
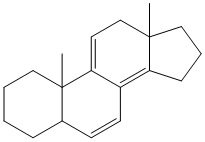 Note- In this example the molecule contains both, a homoannular diene system and a heteroannular diene system. In such a molecule the core chromophore is considered to be the homoannular system and accordingly the calculations are performed.
Note- In this example the molecule contains both, a homoannular diene system and a heteroannular diene system. In such a molecule the core chromophore is considered to be the homoannular system and accordingly the calculations are performed.
Homoannular system
Example/Sample Problem 6
Example/Sample Problem 7
Example/Sample Problem 8
Note- If you have your own problems please write the IUPAC name in the comments section and I will attempt to solve it and add it to this list of examples.
Example/Sample Problems For β-Carotene and all-trans-lycophene
β-Carotene and lycophene are polyenes with more than 4 double bonds, and hence their calculations cannot be done using the Woodward rules. In order to calculate their λmax you must use the Fieser-Kuhn rules. Check out this post on the Fieser-Kuhn Rules to Calculate Wavelength of Maximum Absorption (Lambda-max) of Polyenes (with Sample Problems) for the solution of these problems.
Books on Analytical Chemistry and Spectroscopy
Check out these good books for analytical chemistry and spectroscopy


Ex 8 me jo other effect 30 ×2 kiya h vo kyo kiya gaya h pls explain and what is 35 double bond extending what is the difference in these two limits
Hi Arti, I have updated the table so that the rows align better to the values. Sorry for any confusion. Hope this helps.
can someone plz help me with calculating caffeine??? plz show me how you did it I know its lambda max is 274nm
please provide the method to find out lambda maximum for the following compound..
5-Ethylidene-6-methyl-1,2,3,4,5,6-hexahydro-naphthalene
6,7-Dimethyl-5-methylene-1,2,3,4,4a,5,8,8a-octahydro-naphthalene
You cannot use these rules for aromatic compounds.
Can Eremophilone and allo Eremophilone be discerned by UV Spectroscopy?
Yes but the Woodward-Fieser rules for those are followed by the Conjugated Carbonyl Compounds systems. If you click on the link that article will describe cases like eremophilon and allo-eremophilone. Also check out problems 7 and 8 for similar conjugated carbonyl compounds and how to calculate the wavelength of maximum absorption for that.
In problem #4 double bond extending conjugation should be 2….
How it is 1 ?
Can you please explain?
It is counted as the diene (2 double bonds) + 1 double bond extending conjugation = total of 3 double bonds, so there is only 1 double bond extending conjugation.
I have shown two arrows as you can consider the diene on either side and the 3rd double bond would become the “double bond extending conjugation”. I hope that makes it clear?
what will be the lambda max calculated value for the 2,3-dimethylene [2,2,1] heptane?
please can you calc for anthracene
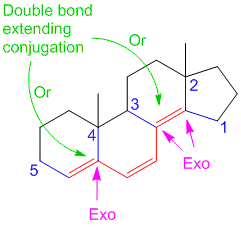
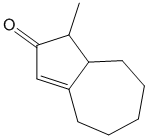
why on “Example/Sample Problem 4” have 3 “exocyclic”?
One is for the first ring and another 2 are for both 6membered & 5membered ring.
Very helpful
Thanks a lot
Can you calculate for benzoyl derivatives?
if OCOCH3 have zero contribution why to be mentioned specifically in the rules.does this have any explanation.
how can i calulate the lamda max of para amino acetyl phenol (paracetamol)?
I want to ask that can u plz give me one example which have Rendo as well as R exo too…?
Sir I want to know the lambda max of halosulfuron methyl herbicide .
How can I calculate maximum wavelength of absorption of sterols especially coprastanol?
Coprastanol has no double bonds, therefore, I doubt it will absorb UV radiation.
i would i know double bond extended conjugation
Akul
I want to ask what if there is comp with formula C8H12O what will its lambda max
Just based on molecular formula it is difficult to predict the lambda max. You need to know structure and extent of conjugation of double bonds.
sir how much base value of acyclic diene
It is the same as a linear diene. Please check the other article.
explain the lamda max value for menthone
Thnk u vry much fr examples… I’ve a problem if anyone can solve pls…. What if two rings are attached with a single bond hetero or homo? like two cyclohaxene rings
Thanks A Lot!
some more exampules
Is an exocylic to 5 membered cyclic ring if it is present in benezene?
No, benzene and other aromatic compounds have different rules.
Thanks here I got my solution….. Nice examples given
sir i want still some more problems related to woodwordsfisher rule
Hi Supritha,
The problems illustrated above are representative examples which cover most scenarios you will find in problems else where. As for practice problems, look for certain text books.
Hi.. akul I’m very proud with your help… may you please send me your email adress in such a way that, we can share different knowledges concerning chemistry?
Hi Ayoub, thanks for the compliments. Unfortunately I cannot share my email id publicly due to security reasons.
thnkyuuuu so much awsom notes…………….
can you help with problems with their solutions involving homocyclic diene component?
Hi Ojeaga,
Please provide the IUPAC name of the molecule you want me to help you with.
Double bond extending conjugation what is mean of this
A double bond extending conjugation means a double bond which is spaced one carbon atom away, such that the electron cloud of the double bond would overlap with the other double bonds in the molecule. The double bond is such that via resonance the electrons can move across the molecule. Check out sample problem 4 for the simplest example.
Hi ,akul this is very useful ………
Hey akul I am confused about double bond extending conjugation…can you please explain??
Hi azakali,
double bond extending conjugation means that there is a double bond which can have a resonance effect on the existing Woodward core component. Take a look at example/sample problem 4 where there are two possibilities of double bond extending conjugation, or in sample 5 where there is one. In all cases, substituents on the double bonds extending the conjugation must also be counted. Please read the original article on the Woodward Fieser Rules here.
pls post few more examples.
Hi Mindak,
At this time we are looking to add more articles rather than just expanding this particular article. This post covers most types of examples you will see. However, if you have a specific problem, please feel free to post in the comments section and if we feel it needs diagramatic explanation, we will add it to this post.
thx foe useful examples
thx for useful examples
this is really very helpful…….thanx
Akul,
These articles are very useful.
Can you apply these rules to 1,4-Benzoquinone?
Best Regards,
Daniel
Hi Daniel,
Sorry for the delayed response and thank you for the compliment. I am happy that this post is useful for you.
As for your question. I do not think you can apply these rules for benzoquinones. But I could be wrong. At least I have never come across it.
The reason I would not think it is possible is because benzoquinones would have pi-electron delocalization similar to aromatic rings. Ultimately it is the delocalization of the pi electrons which controls the UV absorbance. If you do come across any information regarding this matter please do post it as a comment here. It will help others.
Thanks.
This is incredibly helpful! I have a quiz in organic structure tomorrow and this has explained everything I was still wondering about with woodward feiser so thanks for helping me out.
Hi, Akul Mehta
I read some of your articles. It is great to understand analytical chemistry.
I really appreciate your easy and helpful explanation.
I would like to ask one further question.
Extended from Woodward-Fiser rules and Fieser-Kuhn rules,
Is there any rules for complex structure to calculate ?max?
For example,
http://www.rsc.org/ej/EE/2009/b901238a/b901238a-f1.gif
Thanks. I am not so sure that there are any rules for more complicated structures. If I come across anything I will definitely post it here.
Hi Akul,
arent there 2 akyl groups at the beta position in sample question number 8?can u please clarify?
Hi Nimisha,
there is only one alkyl group highlighted in green. The other single bond is attached to a double bond and is hence a part of the double bond extending conjugation (shown in blue) – hence it cannot be counted as an alkyl group. Hope that helps.
Hi Akul Mehta
I read through some of your articles, and find them very good!
Thank you for clear and easy explanations on these topics about abs/fluor.
I would like to ask you if you know how Woodward-Fieser’s rules apply to macromolecules such as proteins? Fx is it possible to apply the rules in order to get a approximation on the chromophore of GFP?
Best regards
Christian
Hi Christian,
Unfortunately the woodward fieser rules I have discussed cannot be applied to aromatic chromophores. Hence they cannot be applied to proteins such as GFP. These rules are only for polyenes and alpha-beta-unsaturated ketones. There are some other rules for aromatic molecules which I might discuss in the future. You can also use online tools available from ExPASy or Uniprot to calculate the absorbance of certain protein sequences but I am not sure how well they would handle unknown chromophores like that in the GFP (4-(p-hydroxybenzylidene)imidazolidin-5-one).
In example 8 I am confused due to exocyclic double bonds. I thought there are 2 EC Double Bonds. One is in Ring A while the other is in Ring B with respect to ring C. Plz clarify it.
Hi Nisar,
By definition, exocyclic double bond is a double bond where one of the participating carbon atoms is a part of a ring, while the other carbon atom is not part of the same ring (see Woodward Rules for conjugated dienes). The one in ring A is simple and it is exocyclic. However, the one between ring B and C is not exocyclic as both carbon atoms are a part of both the rings and hence does not satisfy the definition of only 1 carbon atom being part of the same ring.
Hope this helps.
thanks a lot….very informative
i learned a lot from Ur examples…u make it easy…..thnx and keep it up
thanks alot Mr. mehta. your material is very
helpfull for me.
Hi,in problem 5 :
there shouldn’t be an extended conjugation double bond with the diene system,should be?
Hi Nihal,
In problem 5 there is only 1 double bond extending the conjugation, the other double bonds are a part of the core homo annular diene system. Hope that solves your issue.
Hey Akul
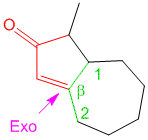
Can you please explain how do we assume the exocyclic double bonds in diene system??
for ex. in problem no. 5 how did we assumed exocyclic bond to be three????
Hi Ash,
Consider labelling the rings from left to right of the steroidal scaffold as A, B, C, D. The exocyclic double bond on top is exocyclic with respect to ring B only. On the other hand the middle double bond is exocyclic to two rings – B and D. The lowest double bond is not exocyclic to any ring. Hence the total exocyclic property is 3. I hope this answers your question.
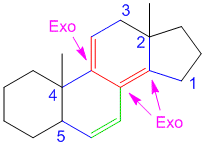
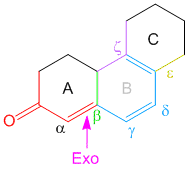
If that’s the case then shouldn’t there be 2 exocyclic bonds in problem 3.
like if we consider rings from left to right say A ,B and so on.If B is exocyclic to A then shouldn’t be A exocyclic to B?? there should be two exocylic bonds or not???
In problem number 3 consider from left to right the rings are labelled A,B,C,D. There are two double bonds: the left one and the right one. By definition, an exocyclic double bond is one which is directly attached to the outside of the ring (i.e. one of the carbon atoms is directly attached to a ring while the other carbon atom is not part of the same ring) (read the description in the basics of woodward fieser rules here). By that definition only the right double bond is exocyclic as it has one of the carbons part of ring A while the other carbon atom of the double bond is actually a part of ring B. For the double bond on the left, both carbon atoms belong to ring A. I hope this makes it more clear. By the way in my previous comments when I say label the rings, I mean’t label the rings and not the double bonds. You cannot say that ring B is exocyclic to ring A.
Thanx a lot !!
got it now…actually i had query related to IR spectra. How would +I and -I affect the C=O bond ???
And thanx a lot for help!
Hi Ash,
Sorry for the late response. But you will have to be more specific as to your IR problem.
Regads.
i have a nmr problem please take a look and reply.
Que.: In a given organic compound three kinds of protons produces signals at 25Hz ,60Hz and 100Hz using 23MHz instrument calculate ? (delta) and tau value.
Hi Azhar,
although this problem is not related to UV-Vis spectroscopy, I can advise you to read the article on Chemical Shift at NMRCentral. In that they have an equation to calculate the delta value (in ppm). Here you substitute the frequency of TMS with the frequency of the instrument as that is the base frequency of TMS on that instrument. I hope this helps.
Thanks a lot! This article made my concepts quite clear…..Initially I was really scare of attempting any question related to this topic but now I can do it quite confidently…:)
Sir, please tell me the detailed solution of calculation of wavelength max of 3,5-dimethylhexa-1,3-diene paying special attention on the number of alkyl groups to be included in the calculation….thnx
Hello sir. Could you please tell me that how come there are 2 alkyl groups in3-methylhepta-2,4,6-triene??
how are cis and trans stilbenes distinguished by Uv spectroscopy
Hi akul,
thanks for the explanations it really helped but what is the wavelength maximum for cyclohex-2-en-1,4-dione., will either of the C=O be assumed to be a double bond extension
Yes… I suppose you could consider either of the carbonyls as a substituent on the ?,?-unsaturated ketone. It depends on what further substituents are there.
In your question number 5.
1. Can you tell me how can green line be the Extended conjugation?
why you cant take this as a ring residue?
2. If -CH=CH2 is attached to 3rd carbon on (a) 1,3-butadiene and (b) On ? position of ?,?-unsaturated ketone system then what will be the lambda max. value.?
Hi Sumit,
1) In this case as mentioned in the note, both homoannular and heteroannular ring residues exist. In such cases you only consider the homoannular ring system, and assume the other one to just be a extended conjugation. This has been found to emperically correlate better with the wavelength of maximum absorption.
2) a) 215 + (30) = 245nm as you consider the alkene to extend conjugation.
2) b) unfortunately here I cannot read whether you have written alpha or beta. So however, in both cases I suppose you consider it as a double bond extended conjugation as if you draw resonance structures they will be extending conjugation. So the value should be = 215 + (30) = 245.
pl let me know in detail how to mark alkyl (R) substituents in examples shown above. Also alkyl substituents in lycophene. how to mark these substituents?
Hi Ritu,
You raised a very important question, and in order to answer that I had to write an entire new post. As for marking alkyl substituents see the above examples to learn. It is important to take all substituents on the entire conjugated system.
In case of lycophene since the number of double bonds is greater than 4 you cannot use Woodward Rules. However you can use Fieser-Kuhn rules to calculate the lambda max. Check out this post on the Fieser-Kuhn Rules to Calculate Wavelength of Maximum Absorption (Lambda-max) of Polyenes (with Sample Problems). I have also made the additions above and on other pages regarding this note. Hope this helps.
kindly solve the problem given above and also tell me why there is only 1 exocyclic bond in sample problem 8, there should be two. Thank you.
Hi, I cannot find this exact structure. Please post a link with some text like the others to an image for the same.
hello i want to ask question..
how about this structure?? how to predict maximal wavelength of this stucture????
http://www.pharmgkb.org/images/drugs/PA448320-0.gif
im so confuse because there is no diena, keton or benzena..
please answer.. thank you 🙂
Hi Ina,
The molecule you are talking about is a heterocyclic molecule. You cannot use these Woodward-Fieser rules for the calculation of wavelength of maximum absorbance. However, you can compare the structure to benzene and derive the wavelengths but it will not be very accurate.
If we have a homoannular diene system and an ?,?-unsaturated ketone/ aldehyde which are in conjugation with each other, then which system should be given priority as the parent compound?
Hi Aditi,
the unsaturated ketone/aldehyde would be the parent chromophore, on which you will add 2 double bond extending conjugations and a homo-annular diene component value of 35 or 39nm. See sample problem number 8 above. Refer to the table given on page and scroll below for the homoannular diene part:
https://pharmaxchange.info/2012/08/ultraviolet-visible-uv-vis-spectroscopy-%E2%80%93-woodward-fieser-rules-to-calculate-wavelength-of-maximum-absorption-lambda-max-of-conjugated-carbonyl-compounds/
heya!!
i just want to know how to find out the double bond extending conjugation..in any one of the question given above
thanks!!
See example 4, it has a double bond extending conjugation.
if dere are two hexacyclic rings adjacent tp each other and conjugation is starting as well as ending with ketone groups with one one keto on both rings…in between ketones,,dere are two congugated double bonds..so last keto group should be considered as ring residue or exocyclic double bond???
Hi Manjula. Sorry for the late reply. It is a little difficult to imagine the structure as I have come up with 3-4 different possibilities of your query. Is it possible to find the structure you have a doubt with on Google and then posting that image link in the comment so that I can take a look at what exactly is your query.
Identify the samples below as either cholesta-4-en-3-one. That is sample 1 and cholesta-4,6-diene-3-one. That is sample 2.
Sample 1 show lambda max at 241nm and sample 2 show lambda max at 281nm
Your question is not very clear. I suppose you could use Woodward-Fieser rules to identify them. Check out rules to calculate lambda max for carbonyl compounds to probably find your answer.
In the sample problem 3…. in substituents for alkoxy group u have substituted +6 but in the table it is mentioned that for alkoxy group its zero.. can u verify and tell.
Hi Shruti,
The alkoxy group is +6nm. Please check the page rules for dienes and polyenes
thanks
plz post some questions on titration i.e. on “double titration”,”titration using iodine” !!!
Thanx….Its really helpfull
You are welcome. Remember to +1 on Google or like on facebook to share it with your friends.