Click Here To Get The Powerpoint Presentation
Abstract
A number of diseases like cancer, arthritis and cardiovascular disorders have commonly been linked to inappropriate changes in the cell’s extracellular matrix (ECM).1 Matrix metalloproteinases (MMP), which are an integral part of the extracellular matrix’s enzymatic arsenal, play a major role in most of these diseases.2 Found in the metamorphosing organs of the anuran tadpole matrix, MMPs have come a long way to be known as the major mediators of the ECM.3,4 Recent indications in cardiovascular disorders however, have generated special interest in this increasingly important field.5
MMPs belong to the metzincin family of enzymes that are known to exploit a zinc ion in their active sites. The MMP family of enzymes consists of 28 members with many different physiological and pathological implications.2 These are released in the inactive proenzyme form and can be activated by a number of factors. They normally function by degrading different substrates of the ECM, eventually leading to tissue remodeling. These are regulated under normal conditions by tissue inhibitors of MMP (TIMP), which is an endogenous inhibitor family of four enzymes. Imbalance in the levels of MMP and TIMP plays a major role in the development of pathological conditions.6,7
Cardiovascular conditions such as myocardial infarction, aneurysms, and atherosclerotic plaques have been associated with enhanced activity of MMPs, especially MMP-2 and MMP-9 (also called as gelatinases). This was further confirmed through knockout and over-expression studies in mice. Over-active MMPs have been the major cause of unwanted tissue remodeling in the cardiovascular system, which signals the need for specific MMP inhibitors.8,9,10
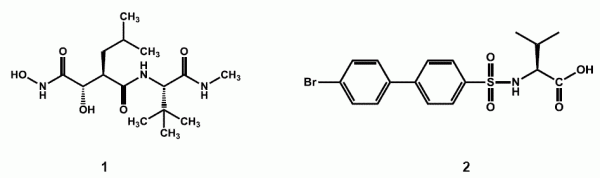
The first generation of MMP inhibitors (1) were based on the substrate collagen and were designed by maintaining the basic protein backbone and replacing the scissile amide bound with a zinc binding group. Many of these agents reached clinical trials as anticancer agents, but the lack of specificity and poor pharmacokinetic profiles of these inhibitors led to a number of side effects.11,12
In the search for more selective agents, the second generation inhibitors were a product of extensive structural activity relationship studies. Major structural advance included replacing the basic protein backbone and a rather constrained structure of the P1’ substituent (Figure 1).13 This led to the design of the sulfonamide type of inhibitors as selective inhibitors of specific groups of MMPs.14 One such compound PG11680 (2) was developed by Parke Davis.15 A selective inhibitor of MMP-2 (IC50= 0.047 uM), MMP-9 (9.9 uM) and MMP-1 (6.1 uM) showed promising preclinical results for preventing Left ventricular hypertrophy associated with myocardial infarction in animals.16 However, clinical trials in humans were unsuccessful as the drug failed to show any significant effect and produced side effects like muscular skeletal syndrome (MSS). Reviewing the causes of failure indicated improper dosing regimens and insufficient selectivity especially, the inhibition of MMP-1 as the major cause of MSS and inadequate response.17
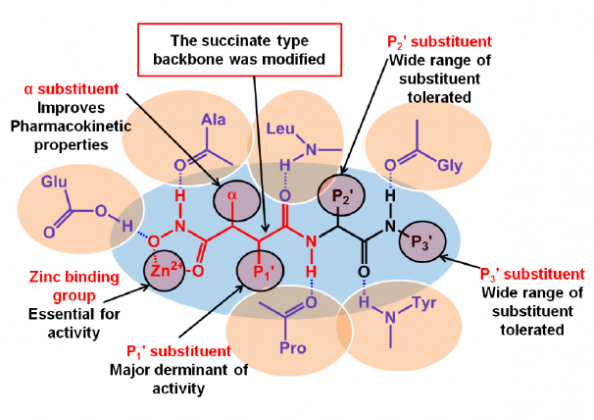
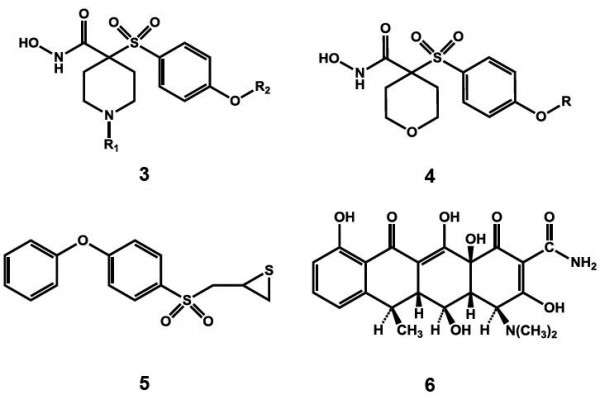
The third generation of MMP inhibitors for cardiovascular disorders specifically aims for MMP-1 sparing activity and selectivity towards gelatinases. Taking advantage of the S1’ selectivity pocket, which has been identified as the only region, which is diverse in various classes of MMPs, a number of agents such as the α-tetrahydropyranyl (3) and α-piperidinyl sulfone (4) have been recently developed by Pfizer.18,19 Novel mechanism-based inhibitors (5) containing thirane moiety has also been developed to selectively inhibit MMP-2.20 An atypical agent for MMP inhibition has also been found in doxycycline (6).21
The implication of MMPs in the cardiovascular disorders is vast and promising however; little is known to understand the actual role of each of the MMPs. A thorough study to understand the role of individual MMPs and improved selectivity through use of the novel strategies therefore, is the need of the hour for this class to succeed from potential agents to a clinically important class.
References
- Brinckerhoff, C. E.; Matrisian, L. M. Matrix metalloproteinases: a tail of a frog that became a prince. Nature Rev. Mol. Cell Biol. 2002, 3, 207-214.
- Page-McCaw, A.; Ewald, A. J.; Werb, Z. Matrix metalloproteinase and the regulation of tissue remodelling. Nature Rev. Mol. Cell Biol. 2007, 8, 221-233.
- Gross, J.; Lapiere, C. M. Collagenolytic activity in the amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. 1962, 48, 1014-1022.
- Gross, J.; Nagai, Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc. Natl. Acad. Sci. 1965, 54, 1197-1204.
- Liu, P.; Sun, M.; Sader, S. Matrix metalloproteinases in cardiovascular disease. C. J. Cardiol. 2006, 22, 25-30.
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta, 2010, 1803, 55-71.
- Spinale, F. G. Myocardial matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol. Rev. 2007, 87, 1285-1342.
- Spinale, F. G.; Coker, M. L.; Bond, B. R.; Zellner, J. L. Myocardial matrix degradation and metalloproteinase activation in the failing heart: a potential therapeutic target. Cardiovasc. Res. 2000, 46, 225-238.
- Creemers, E. E. J. M.; Cleutjens, P. M.; Smits, J. F. M.; Daemen, M, J. A. P. Matrix Metalloproteinase inhibition after myocardial infarction new approach to heart failure. Circ. Res. 2001, 89, 201-210.
- Spinale, F. G. Matrix metalloproteinase: Regulation and dysregulation in the failing heart. Circ. Res. 2002, 90, 520-530.
- Whittaker, M.; Floyd, C. D.; Brown, P.; Gearing, A. J. H. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem. Rev. 1999, 99, 2735-2776.
- Brown, P. D. Matrix metalloproteinases inhibitors in the treatment of cancer. Medical Oncology 1997, 14, 1- 10.
- Hu, J; Van den Steen, P. E.; Sang, Q. A.; Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular disease. Nat. Rev. Drug Discovery, 2007, 6, 480-498.
- Kontogiorgis, C. A.; Papaioannou, P.; Hadjipavlou-Litina, D. J. Matrix metalloproteinase inhibitors: A review on pharmacophore mapping and (Q)Sars results. Curr. Med. Chem. 2005, 12, 339-355.
- O’Brien, P. M.; Ortwine, D. F.; Pavlovsky, A. G.; Picard, J. A.; Sliskovic, D. R.; Roth, B. D.; Dyer, R. D.; Johnson, L. L.; Man, C. F.; Hallak, H. Structure-activity relationships and pharmacokinetic analysis for a series of potent, systemically available biphenyl sulfonamide matrix metalloproteinase inhibitors. J. Med. Chem. 2000, 43, 156-166.
- Kaludercic, N.; Lindsey, M. L.; Tavazzi, B.; Lazzarino, G.; Paolocci, N. Inhibiting metalloprotease with PD- 166793 in heart failure: Impact on cardiac remodeling and beyond. Cardiovascular Therapeutics, 2008, 26, 24–37.
- Hudson, M. P.; Armstrong, P. W.; Ruzyllo, W.; Brum, J.; Cusmano, L.; Krzeski, P.; Lyon, R.; Quinones, M.; Theroux, P.; Sydlowski, D.; Kim, H. E.; Garcia, M. J.; Jaber, W. A.; Weaver, W. D. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction. J. Am. Coll. Cardiol. 2006, 48, 15-20.
- Devel, L.; Czarny, B.; Beau, F.; Georgiadis, D.; Stura, E.; Dive, V. Third generation of matrix metalloprotease inhibitors: Gain in selectivity by targeting the depth of the S1’ cavity. Biochimie, 2010, doi:10.1016/j.biochi.2010.07.017. (article in press)
- Becker, D. P.; Barta, T. E.; Bedell, L. J.; Boehm, T. L.; Bond, B. R.; Carroll, J.; Carron, C. P.; DeCrescenzo, G. A.; Easton, A. M.; Freskos, J. N.; Funckes-Shippy, C. N.; Heron, M.; Hockerman, S.; Howard, C. P.; Kiefer, J. R.; Li, M. H.; Mathis, K. J.; McDonald, J. J.; Mehta, P. P.; Munie, G. E.; Sunyer, T.; Swearingen, C. A.; Villamil, C. I.; Welsch, D.; Williams, J. M.; Yu, Y.; Yao, J. Orally active MMP-1 sparing α-tetrahydropyranyl and α-piperidinyl sulfone matrix metalloproteinase (MMP) inhibitors with efficacy in cancer, arthritis, and cardiovascular disease. J. Med. Chem. 2010, 53, 6653-6680.
- Ikejiri M.; Bernardo, M. M.; Bonfil, R. D.; Toth, M.; Chang, M.; Fridman, R.; Mobashery, S. Potent mechanism based inhibitors of matrix metalloproteinases. J. Biol. Chem. 2005, 280, 33992-34002.
- 21. Franco, C.; Ho, B.; Mulholland, D.; Hou, G.; Islam, M.; Donaldson, K.; Bendeck, M. P. Doxycycline alters vascular smooth muscle cell adhesion, migration, and reorganization of fibrillar collagen matrices. Am. J. Pathol. 2006, 168, 1697-1709.

hi
thanks for your Interesting Post
If it is possible
Please mail to me PDF