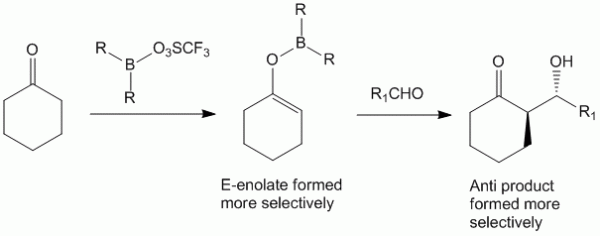
Synthesis of highly selective enol in return governs the selectivity of the product obtained in aldol synthesis as was observed in the case of Directed Aldol Synthesis chapter. Preparation of boron enolates offers an advantage of obtaining highly selective enolate to control aldol condensation.
Boron enolate may be formed by reacting the carbonyl compound (containing α-hydrogens) with the triflic salt of the dialkyl boron compound in the presence of base such as diisopropyl ethyl amine.

The formation of the enolate is more selective in case of Boron as compared to Lithium salts due to two reasons:
- The boron-oxygen bonds are shorter, therefore the cyclic transition state formed is more compact and rigid thus allowing the substituents to play a greater part in the stereocontrol.
- The substituents on the boron atom (R) can be made very bulky in order to control selectivity for the enolate formed.
Therefore, the stereochemistry of enolization is influenced by:
- The alkyl groups on the boron atom and their bulkiness.
- The leaving group on the boron (in the case above it is triflate).
- The base involved in the reaction.
Example of Stereoselective Aldol Condensation Using Boron Enolate
A good example of stereoselectivity imparted in aldol synthesis using boron enolates involves the aldol condensation of cyclohexanone. When cyclohexanone is reacted with alkylated boron triflate, it forms selectively the E-enolate yielding the anti product in greater yields.
Books on Organic Chemistry
Check out some of the best textbooks for Organic Chemistry
References
- Advanced Organic Chemistry: Reactions and synthesis. By Francis A. Carey, Richard J. Sundberg
- D. A. Evans, E. Vogel, J. V. Nelson, J. Am. Chem. Soc., 1979, 101, 6120.
- Brown J. Am Chem. Soc. 1989, 111, 3441.
- Brown J. Org. Chem. 1993, 58, 147.