Introduction
Most pharmaceutical drugs and xenobiotics contain aliphatic or alicyclic functional groups within their structure. Oxidation at aliphatic and alicyclic positions in molecules via mixed-function oxidases is one of the most common modes of metabolism for pharmaceutical drugs and xenobiotics. Oxidative metabolism of aliphatic and alicyclic molecules involves the addition of a hydroxyl group to a methyl group. It is important to know the difference between aliphatic and alicyclic systems.
Note- Please check our parent article on metabolism of pharmaceutical drugs and xenobiotics for other metabolic pathways.
Aliphatic compound: Carbon atoms joined together in straight chains or branched chain. These carbon chains can be saturated i.e. joined by single bonds (alkanes), or unsaturated i.e. with double bonds (alkenes) or triple bonds (alkynes). Apart from hydrogen, other elements can be bound to the carbon chain, the most common being chlorine, nitrogen,oxygen,and sulfur.
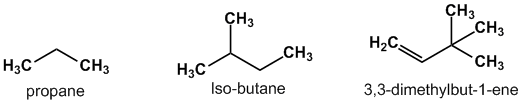
Alicyclic compound: These compounds consist of carbon atoms that link together to form a ring structure. They are thus aliphatic and cyclic in nature. The carbon atoms are joined in the form of a ring with the presence of either single (saturated) or (unsaturated) bonds. They do not exhibit any aromaticity.
Metabolism via Aliphatic Oxidation
Rule of thumb: Aliphatic oxidation generally occurs in molecules containing branched or straight aliphatic carbon chains at ‘ω’or terminal carbon atom and/or ‘ω-1’ or penultimate carbon atom position.
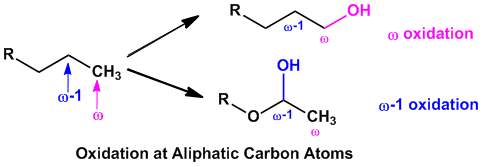
Depending upon which carbon atom in the aliphatic compounds is undergoing oxidation, variable products are formed.
- When enzymatic oxidation takes place at the terminal carbon atom, known as ‘ω’ oxidation it results in hydroxylation of the terminal carbon atom. In some cases, when enzymatic oxidation takes place at the penultimate (carbon atom next to the terminal carbon) carbon atom, known as ‘ω-1’ oxidation, forming alcohol metabolites.
- These type of oxidations (‘ω’ and ‘ω-1’ ) mostly happens with straight chain or branched aliphatic hydrocarbons.
- The initial metabolites formed from ‘ω’ or ‘ω-1’ oxidation both further can either be oxidized to aldehydes/ketones or carboxylic acids, or they may undergo glucuronide conjugation.
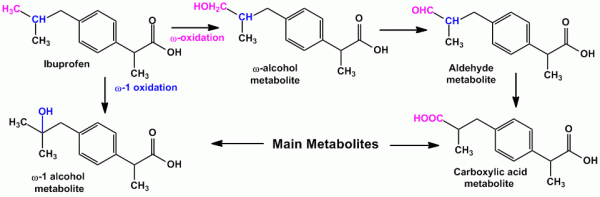
- For isopropyl side chain, oxidation occurs at the tertiary carbon atom yielding carboxylic acid and at either of the equivalent methyl group yielding tertiary alcohol metabolites. Eg. Ibuprofen (an anti-inflammatory agent) undergoes both ‘ω’ and ‘ω-1’ oxidation yielding carboxylic acid and tertiary alcohol metabolite respectively.
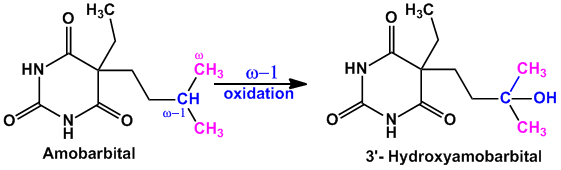
- Example of compounds undergoing majorly ‘ω-1’ oxidation are Amobarbital (sedative hypnotic agent), Chlorpropamide (oral hypoglycemic agent)
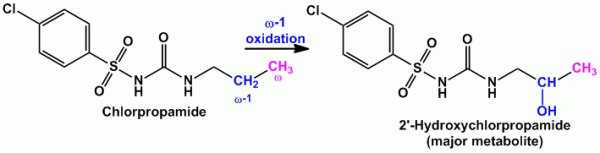
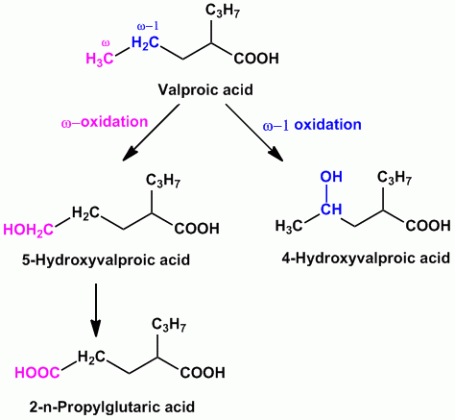
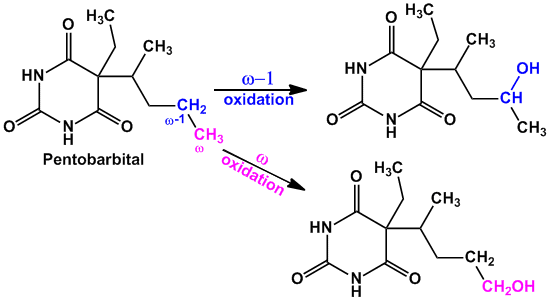
- Example of compounds undergoing both ‘ω’ and ‘ω-1’ oxidation are Valproic acid (anti-epileptic agent), Pentobarbital (sedative hypnotic), Thiamylal (barbiturate- sedative hypnotic), Secobarbital (barbiturate- sedative hypnotic) etc.
- Other compounds which get metabolized by aliphatic hydroxylation are Glutethimide (sedative hypnotic), Meprobamate (anti-anxiety), Ethosuximide (anti-epileptic) etc.
- The main metabolic pathway for the methyl group attached to aromatic rings is oxidation to hydroxyl group forming hydroxymethyl derivative. In other cases the hydroxymethyl is further oxidized to carboxylic acid. eg. Tolbutamide (anti-diabetic)
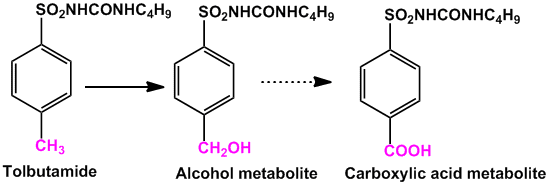
- When there are more than one equivalent methyl groups, usually only one of the methyl group undergoes oxidation. Para position is likely to undergo oxidation in cases of aromatic methyl groups.
- When the alkyl side chains are attached to an aromatic ring, the aromatic ring influences the oxidation of the alkyl side chain. Though in most cases, oxidation takes place majorly at the benzylic methylene group and in lesser extents at other positions of the side chain.
Metabolism via Alicyclic Oxidation
Rule of thumb: In cases of presence of monosubstituted cyclohexyl ring, oxidation (hydroxylation) occurs either at C-3 or C-4 position and can also result in formation of cis and trans products (see figure below).
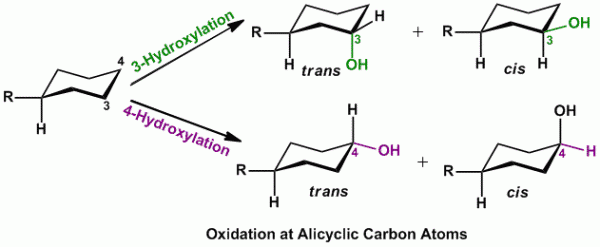
- Alicyclic oxidation is carried out by mixed function oxidases.
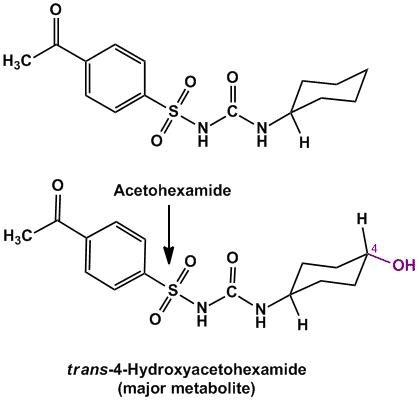
- Example of compounds undergoing alicyclic oxidation are Acetohexamide (oral hypoglycemic agent), Glipizide (oral hypoglycemic agent)
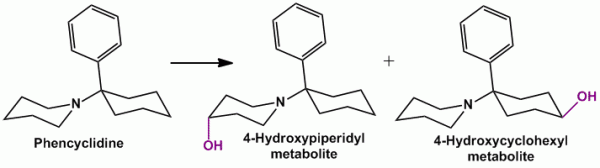
- For a piperidyl ring, it resembles oxidation (hydroxylation) of a cyclohexyl ring, thus getting oxidized at C-4 position. Example, Phencyclidine (formerly used as an anesthetic), Minoxidil (antihypertensive agent)
- The methylene groups attached to an alicylic ring undergo oxidation (hydroxylation) generally at the most activated position or at the least hindered position. In cases of a cyclohexanone ring (α- to a carbonyl), a cyclohexene ring (α- to a double bond) and a phenyl ring (eg.tetralin) oxidation occurs at the alpha position as discussed under oxidation at carbon atoms α to carbonyls and imines, or amines or allylic systems.
References
1. Foye’s Principles of Medicinal Chemistry (Buy at Amazon.com)
2. Wilson & Grisevold’s Textbook of Organic Medicinal Chemistry. (Buy at Amazon.com)