Introduction
Paracetamol (acetaminophen), acetanilide and phenacetin belong to a class of antipyretic analgesics. Acetanilide (antifebrin) was the first to have been introduced in the year 1886, but its use was limited at high doses due to toxic side effects of methemoglobinemia and jaundice. Phenacetin was released in 1887 and was in use until recently, when reports of nephrotoxicity caused its removal. Due to its metabolite (acetaminophen), phenacetin has a long duration of action. However it was a weaker antipyretic as compared to paracetamol. Paracetamol (acetaminophen) was prepared in 1893. However, it took nearly 50 years for scientists to realize that acetaminophen is the active metabolite of both acetanilide and phenacetin. It was only after this realization that it’s use gained popularity. A lot of the activity and the side effects of these drugs can be attributed to their metabolites and hence we take a closer look at their metabolism in the body.
Metabolism of Paracetamol, Acetanilide and Phenacetin
The metabolism of acetaminophen (paracetamol), phenacetin and acetanilide are highlighted in the figure below. In this figure shades of red highlight potential toxic substances, while green highlights non-toxic products which can be excreted safely by the body.
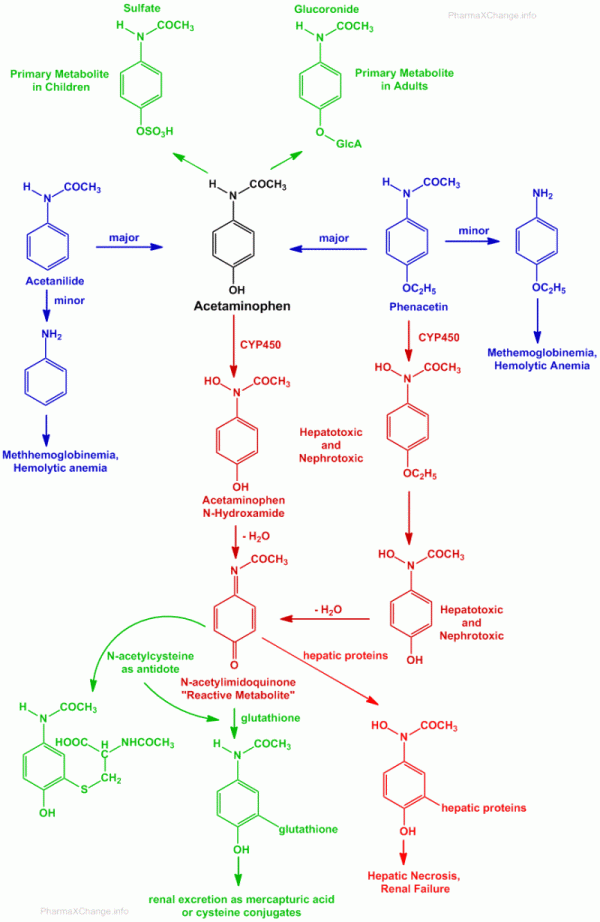
- As highlighted earlier both acetanilide and phenacetin are prodrugs of paracetamol.
- Both phenacetin, as well as acetanilide undergo hydrolysis to yield aniline derivatives.
- These aniline derivatives can cause methemoglobinemia and hemolytic anemia.
- The primary metabolism routes for paracetamol (acetaminophen) are by O-conjugation to yield O-sulfate conjugate primarily in children and O-glucuronide conjugate primarily in adults.
- Paracetamol and phenacetin may also undergo oxidative metabolism via cytochrome P450 (CYP450) mixed function oxidase systems, to yield N-hydroxyamide products.
- The N-hydroxyamide products are reactive and can lose a water molecule to produce a reactive N-acetylimidoquinone (an imine)(NAPQI).
- The N-acetylimidoquinone (NAPQI) is believed to be bind to hepatic and renal proteins and cause the hepatotoxicity and nephrotoxicity that is associated with paracetamol and phenacetin.
- Under normal conditions this reactive N-acetylimidoquinone is conjugated in the liver with the help of glutathione which can then be excreted as mercapturic acid or cysteine conjugates.
- In case of over dose of paracetamol, the glutathione reserves may be depleted due to conjugate formation. Above 70% of this depletion, the reactive N-acetylimidoquinone metabolite will begin to react with nucleophilic thiol (-SH) groups that are present on hepatic and renal proteins. This results in formation of covalent adducts which produce hepatic necrosis and renal tubular necrosis.
- Overdose of paracetamol can result in severe hepatic necrosis and renal failure to the extent of fatality.
- Sulfhydryl-containing compounds can be useful as antidotes for such overdose related toxicity of paracetamol. Out of all sulfhydryl containing molecules, N-acetylcysteine is the most widely used for paracetamol toxicity.
- N-acetylcysteine serves three purposes towards the antidote treatment in case of paracetamol poisoning and overdose: (i) serves as a substitute for depleted glutathione (ii) N-acetylcysteine being the precursor of glutathione, can help in replenishing the lost glutathione reserves (iii) it can itself react with the N-acetylimidoquinone to neutralize the threat and excrete it safely from the body. It is also believed that the N-acetylcysteine can help reduce the formation of the N-acetylimidoquinone metabolite itself.
References
- Borne, R. F. Nonsteroidal Anti-inflammatory Agents. In: Foye’s Principles of Medicinal Chemistry 6th Edition; Williams D. A.; Lemke T. L., Lippincott Williams & Wilkins Philadelphia, 2007; p751-793.
(Buy at Amazon)|(Buy at AbeBooks.com)
- Prescott, L. F. Paracetamol (Acetaminophen): A Critical Bibliographic Review. CRC Press, 1996.
(Buy at Amazon)|(Buy at AbeBooks.com)

The article does not mention that paracetamol toxicity is a threshold effect: For example, for an average subject, not drinking alcohol, a dose of 4 g in 24 hr. may be safe, while 4.5 g is toxic. These figures are about 1/2 if the subject is drinking. It would be helpful to know the dose of the antidote NAC (N-acetylcysteine), as this is widely available as a supplement in health stores and could be in the family first-aid chest. I believe the clinical dose as antidote is 3 g, I’m not sure about repeat dosing — need expert help here.
The popular painkiller acetaminophen (also known as paracetamol) may cause rare but serious skin reactions, the Food and Drug Administration (FDA) has warned. The analgesic is one of the most commonly and long-used drugs available, sold under numerous brandnames in the US.
This article has been helpful for my assignment on paracetamol. I’ll be sure to reference it. Thanks.
We greatly appreciate it. Be sure to +1 on Google or share on Facebook.