What is a Thermodynamic Product and a Kinetic Product?
A chemical reaction in which more than one product can be formed is generally governed by either laws of thermodynamics or kinetics. It can therefore yield either thermodynamic products or kinetic products.
Imagine a reaction in which there are two products which can be formed i.e. either product A or product B.

The energetics of the reaction may be written as follows:
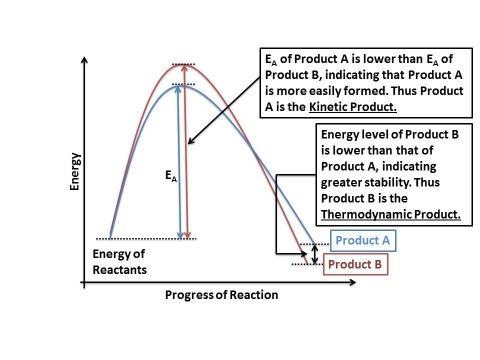
In case of product A, the energy of activation (EA) is lower than that of product B. Which means that the amount of energy required to produce product A is lesser, and hence product A will form faster than product B. Due to this factor, product A is known as the kinetic product of the reaction.
In case of product B, the energy of activation (EA) is higher than that of product A. Which means more energy would be spent in making product B, and hence it would be formed slower. However, once product B is formed, the overall energy of the molecule is lower than that of product A. This means that the stability of B is greater than that of A. The more stable product, i.e. product B, is known as the thermodynamic product of the reaction.
Examples
2-Methylcyclohexanone
When 2-methylcyclohexanone is an asymmetric ketone. When it is exposed to basic conditions, it can undergo enolate formation on either side of the molecule to produce either kinetic product (A) or thermodynamic product (B).
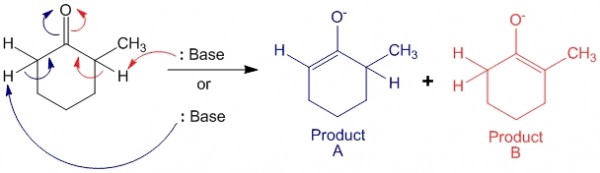
In this case product A is the kinetic product, as the base can abstract the less hindered proton (at position 6 of the compound) more easily as compared to the proton at position 2 (to form product B). Also, product B is the thermodynamic product since the alkene formed is a more substituted alkene – enolate ion. It is known that the more substituted an alkene is, the more stable it is.
Case 1: In presence of strong base such as LDA:
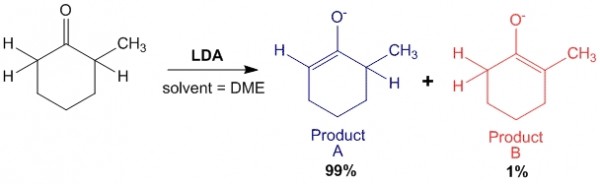
In this case, LDA being a strong base abstracts the least hindered hydrogen with greater ease, hence forming a majority of Product A which is the Kinetic Product of the reaction.
Case 2: In presence of weak base such as triethylamine.
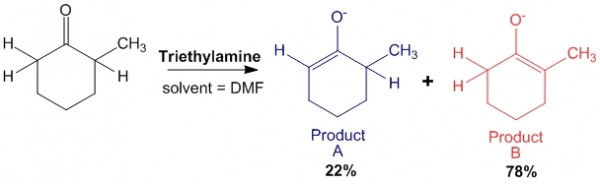
In this case, triethylamine is a weak base. Therefore, when it abstracts the alpha-hydrogen on the less hindered side, the reaction is a reversible reaction. Thus there will be an equilibrium between the reactants and products.
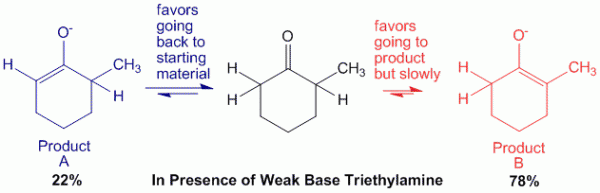
This allows enough energy to be obtained for the base to abstract the other proton on the other side of the molecule to produce product B, which is the thermodynamic product.
Getting Selectivity for either Thermodynamic Product or Kinetic Product
Kinetic products are in general, more easily formed than thermodynamic products due to the lower energetics required. Since to form a thermodynamic product, you will have to supply enough energy to the system which is greater than that for the kinetic product, you will always get some of the kinetic product in the reaction. But there are some tricks you could use while doing these reactions in order to produce more of either
To Get Kinetic Product:
A kinetic product is favored under non-equilibrium conditions.
- Shortening the reaction time – this will ensure that there is not enough time for a reversible reaction to occur to lead to the formation of more stable thermodynamic product.
- Use of strong base (or strong reagents) – This will ensure that the process is not reversible at the intermediate step.
- Try doing the reaction at lower temperatures – that way you do not provide more energy to cross the EA of the thermodynamic product. But keep in mind, that lowering the temperature may also allow equilibrium conditions to make the reaction reversible and hence allowing formation of thermodynamic conditions.
- Use of hindered base – This point refers to the above example of 2-methylcyclohexanone – where abstraction of less hindered proton would result in formation of kinetic product. If therefore, we use a hindered or bulky base, the more hindered proton would not be readily taken up by the base, thus pushing the kinetic control into the reaction.
To Get Thermodynamic Product:
In order to get thermodynamic control, you need conditions of reversibility at some stage of the mechanism. If that exists then you can try the following:
- Lengthening the reaction time – longer reaction times will ensure that the equilibrium favors formation of more stable product
- Use of milder base (or milder reagents) – This will ensure a greater equilibrium stage allowing reactions to occur in a reversible manner in the mechanism.
- Use of higher temperatures
Books on Organic Chemistry
Check out some of the best textbooks for Organic Chemistry
References
- Organic Chemistry. Second Edition. By Thomas N. Sorrell – Chapter 22.
- Advanced Organic Chemistry: Reactions and synthesis. By Francis A. Carey, Richard J. Sundberg

Extremely helpful! Now I’m hoping there will be thermo vs. kinetic question on my exam 🙂
Very helpul information
Thanks man , I was needing these information necessarily 🙂
????? ??????
Very useful info. Well presented. Kudos.
Thank you for your post!
You’ve been really helpful^^*
I really appreciate how concise and helpful this was!!!! Thank you for putting this up
Hello, glad to hear that it helped you. If you liked the post do remember to +1 and share it. Also do check us out on facebook and other social media to get the latest updates.