Acetoacetic ester synthesis is similar to malonic ester synthesis.When β-keto ester is reacted with a weak base such as Sodium Hydroxide (NaOH) in the presence of alkyl halide (R-X), it produces α-alkylated β-keto esters and also di-alkylated products. However, upon acidification the mixture will lose a molecule of carbon dioxide to get decarboxylated to give ketones as well. This will be further explained with the mechanism.
Mechanism of Acetoacetic Ester Synthesis
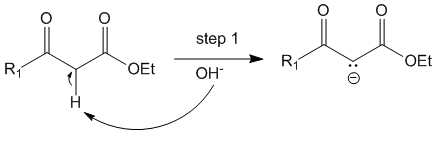
Step 1
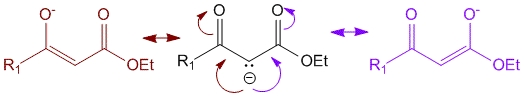
In the presence of mild base such as NaOH or NaOEt, the alpha – hydrogen of the beta-keto ester can be abstracted to produce the carbanion which can resonate to form an enolate. Since the enolate can resonate on either side of the ester and the ketone it is more stabilized and hence use of even mild bases can be made for this reaction.
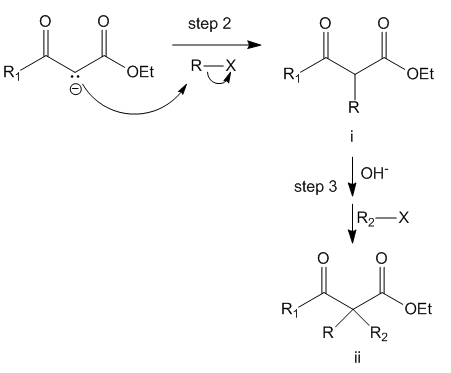
Step 2 and Step 3
The enolate carbanion thus formed is capable of attacking the alkyl halide R-X as the halide is a good leaving group. This yields the mono-alkylated beta-keto ester (i). However, reaction does not stop there. Since the base and alkyl halide are present in the same solution, step 1 and 2 can repeat in step 3 to produce the disubstituted beta-keto ester (ii).
Note – For step 2 the carbanion shown below can exist as enolates in resonance structures (as shown above) – hence it can be shown using the illustration below or using enolates but with proper arrows indicating movement of electrons.
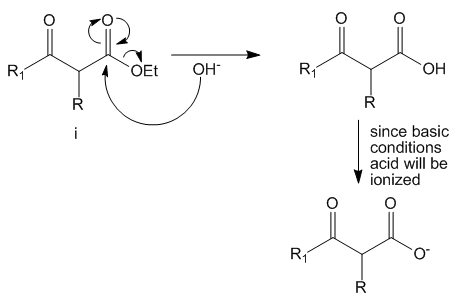
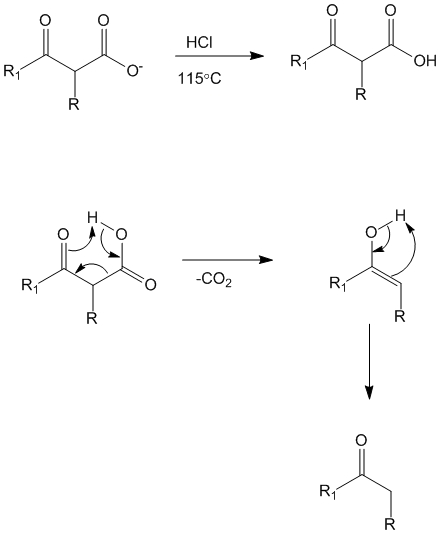
Step 4
Upon acidification of the solution the esters can undergo hydrolysis to yield the acid. Note – this reaction can happen with both mono (as shown) as well as di-substituted beta keto ester (not shown).
Step 5
Under acidic conditions and slight heating the beta-keto carboxylate can undergo a loss of carbon dioxide to yield either mono (as shown) or disubstituted (not shown) ketone.
Disadvantages
Like Malonic Ester Synthesis – acetoacetic ester synthesis is also non-selective and gives low yield for one single product as it can form a plethora of products. This non-selective nature of acetoacetic synthesis can be used to produce cyclic products. Also O- vs C- alkylation competition also exists.
Books on Organic Chemistry
Check out some of the best textbooks for Organic Chemistry
References
- Advanced Organic Chemistry: Reactions and synthesis. By Francis A. Carey, Richard J. Sundberg
- Organic-Chemistry.org (accessed on February 06, 2011)

Great work Akul!