Introduction
Fatty acids are oxidized in the mitochondrial matrix by the enzymes present there. Before oxidation the fatty acids have to go through the process of activation and transportation via the carnitine shuttle through the mitochondrial membrane. Oxidation of fatty acids results in the yield of energy which is twice as much as from oxidation of carbohydrates and proteins. (For further details read: Oxidation of Fatty Acids and Glucose Metabolism). Most of the fatty acids present have even carbon chain length while there are some fatty acids in plants and marine species which have odd carbon chain length.
β-oxidation of Odd Carbon Chain Length Fatty Acids
Just the way even chain carbon length fatty acids undergo β-oxidation (For details read Oxidation of Fatty Acids), odd carbon chain fatty acids undergo the same pathway leaving propionyl CoA as an end product along with acetyl CoA molecules. This propionyl-CoA is formed because during the last β-oxidation cycle the fatty acyl-CoA with five carbons is cleaved to give a three carbon compound and a two carbon compound unlike even carbon chain length fatty acid. Acetyl CoA enters the Kreb’s cycle but propionyl CoA has a different fate.
Propionyl CoA fate is shown below in the diagram.
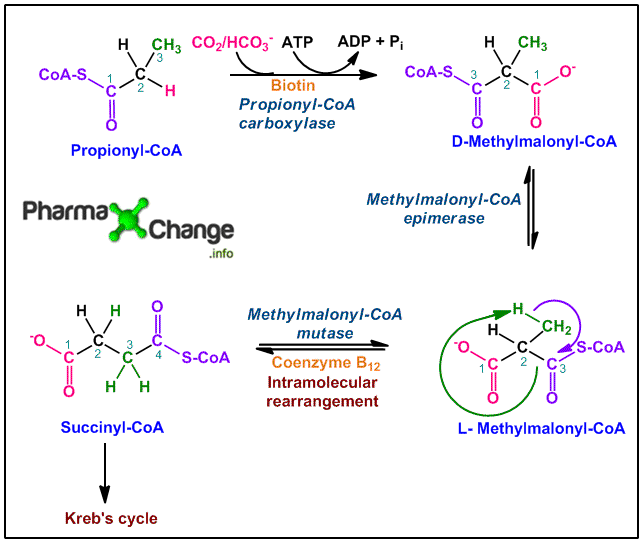
Discussion
- Propionyl-CoA in presence of the enzyme propionyl-CoA carboxylase is carboxylated either by CO2 or HCO3–. This is an ATP dependent step and utilizes one ATP molecule. The enzyme propionyl-CoA carboxylase contains the co-factor biotin, where HCO3– binds. This binding requires energy (ATP) resulting in the production of the carboxybiotin intermediate. This intermediate further progresses to form D-Methylmalonyl-CoA.
- D-Methylmalonyl-CoA is then acted upon by the enzyme methylmalonyl-CoA epimerase or methylmalonyl-CoA rasemase to form the ‘L’ isomer, L-methylmalonyl-CoA. This reaction occurs as it is important, as metabolic pathways in the body occur only with majorely ‘L’ isomer species.
- The ‘L’ isomer species then undergo intramolecular rearrangement catalyzed by the enzyme methylmalonyl-CoA mutase. Intramolecular rearrangement results in the formation of succinyl-CoA. The enzyme participating in this reaction is dependent on coenzyme B12 (5′-deoxyadenosylcobalamin) which plays a crucial role in the reaction. In this intramolecular rearrangement, the —CO—S-CoA group at C-2 position from the original propionate molecule exchanges position with the H atom at C-3 position of the original propionate.
- Succinyl-CoA formed now enters the Kreb’s cycle and gets converted to CO2 and H2O with the release of energy.
Recommended Texts
- David L. Nelson and Michael M. Cox, Lehninger Principles of Biochemistry 6th Edition
- Jeremy M. Berg, John L. Tymockzo and Luber Stryer, Biochemistry 7th Edition
- Reginald H. Garrett, Charles M. Grisham, Biochemistry by Reginald H Garrett 5th Edition
.
- U. Satyanarayana, U. Chakrapani, Biochemistry by U. Satyanarayana. 3rd Edition.