Click Here To Get Presentation
Abstract
Protein-protein interactions are very important because they regulate a vast array of biological processes at the cellular level. Oligomerization of G-protein coupled receptors (GPCRs) is a vital protein-protein interaction under study.1,2 Initially received with incredulity, the concept of GPCR oligomerization is now picking up momentum with increasing data for its existence.3 GPCR oligomers, in contrast to their monomers, show diverse pharmacological as well as receptor properties.4,5 Findings suggest that GPCR oligomerization occurs as early as its maturation. These oligomers could have a whole new way of interacting with G-proteins, the effects of ligands, downstream activation of signaling pathways, and receptor internalization.6 Oligomers have been studied over the years by a number of biochemical and biophysical techniques. Fluorescence based techniques, Förster resonance energy transfer (FRET), bioluminescent resonance energy transfer (BRET), and bimolecular fluorescence complementation (BiFC) are the most widely used techniques.7
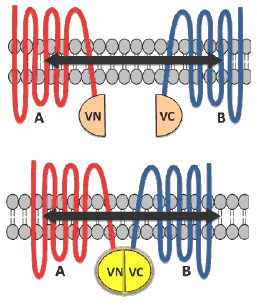
BiFC is a type of protein complementation assay (PCA) that has proved to be a competent method for studying GPCR dimer visualization, localization, and ligand-based effects.8 In principle, the technique involves a fluorescent protein such as Venus (V) which is split into two complementary fragments that are non-fluorescent. The N terminal of Venus (VN) is tagged to protein A and the C terminal of Venus (VC) is tagged to protein B (Figure 1). Complementary sequences of the fluorescent proteins then associate to form a functional complex when the tagged fusion proteins A and B interact. Excitation of this complex with light of an appropriate wavelength results in emission of fluorescence that aids in revealing vital interactions between the proteins under study.1,2,8,9
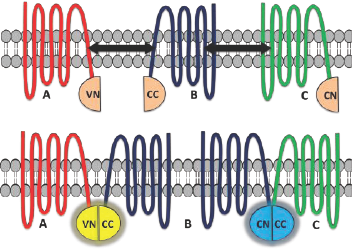
A useful variation of the above assay, multicolor BiFC (MBiFC), allows the simultaneous detection of more than one protein interaction in the same cell.1 In this assay, two different fluorescent proteins such as Venus and Cerulean (C) are used. The N-terminal end of the Venus fragment is tagged to protein A (VN) and the C-terminal end of the Cerulean fragment is tagged to protein B (CC) (Figure 2). A complementary sequence of the N terminal of the Cerulean fragment is tagged to protein C (CN). Protein interactions between the A-B pair and B-C pair can be easily detected due to the reconstitution of the complementary non-fluorescent protein fragments. Employing different excitation and emission wavelengths, each protein interaction can be simultaneously visualized in the same cell.1,2,8
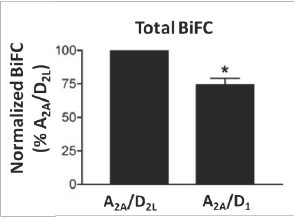
The employment of BiFC and MBiFC has been essential in understanding the existence and the functional effects of GPCR oligomerization. The versatility of this technique can be further appreciated by exploring its utility in a number of GPCR oligomerization assays.10-12 BiFC has been used to demonstrate oligomerization of dopamine D2L and adenosine A2A receptors. The detection of a fluorescence signal indicates dimerization between D2L and A2A receptors (Figure 3). To demonstrate the specificity of the interaction D2L receptor was replaced with a D1 receptor. Dimerization of D2L and A2A receptors shows implications of adenosine A2A antagonists along with dopamine D2L agonists in treating Parkinsonism.11
The usefulness of the MBiFC technique can be readily demonstrated through its applicability tocannabinoid CB1 and dopamine D2L receptor heteromerization, and dopamine D2L homomerization in thesame cell.12,13 The results of this assay implied a role of CB1-D2L heteromers in drug abuse and CB1 antagonists in Parkinsonism.12 Similar uses make MBiFC a powerful technique for simultaneousidentification of multiple interactions.1
The stoichiometry of many GPCR oligomers remains largely unknown.14 A major limitation of PCAs and resonance energy transfer (RET) methods includes its ability to permit the detection of only two interacting proteins, thus highlighting the need for a technique to study the interaction between higherorder oligomers.14,15 The combination of PCAs and RET methods in sequence could offer a path to further allow the detection of higher-order oligomers. The development of BiFC-RET offers a major improvement in the identification and study of the stoichiometry of GPCR oligomers.11,14,16
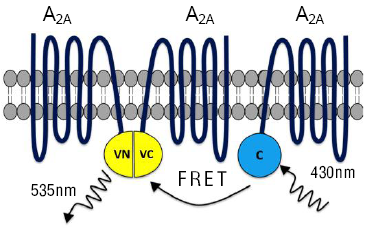
The utility of the BiFC-FRET assay has been adequately showcased through its use in the study of the stoichiometry of adenosine A2A receptors.14 These receptors are known to form higher-order oligomers and the use of the newly developed BiFC-FRET technique makes it feasible to detect trimers of A2A receptors (Figure 4). The assay incorporates the use of Cerulean tagged to adenosine A2A as a FRET donor, and the BiFC pair of Venus-tagged adenosine A2A receptors as a FRET acceptor.10,14
The concept of GPCR oligomerization leads to innovative and challenging opportunities for drug discovery and development for medicinal chemists.17 Drug targets were initially thought of as monomers, but with this new GPCR concept the drug target could be a dimer or a higher-order oligomer.4,5 This would imply alterations in ligand-binding properties, and signaling, of GPCRs which open a whole new world for GPCR-target drug discovery. Their implication in various disease states can also be exploited to potentially produce drugs with increased tissue specificity and decreased side effects.18,19
BiFC has advantages over other fluorescence techniques such as FRET and BRET.8 It allows direct visualization, lower levels of protein expression, relaxed requirements for spatial proximity, and detection of multiple protein interactions in the same cell. A relatively longer fluorophore maturation time and irreversibility of the complementation complex gives BiFC an impediment. 8,9
In addition to GPCR oligomerization BiFC has found utility in studying various other protein interactions that are useful to medicinal chemists. These include chromatin structure assembly, protein interactions in herpes simplex virus, interactions of Alzheimer-associated proteins, and verification of homology models, amongst others.20-23
Improvements in the technique in the form of mutations in the fluorescent protein to improve signal-tonoise ratio of BiFC make the assay more effective.24 The combination of PCA-RET techniques open new avenues of exploration, and the irreversibility of the protein-protein interactions due to BiFC is envisaged to be used as a method to isolate stable protein-protein interactions. It is also proposed that PCAs could be used as a method for drug screening.14 Thus, the future potential of BiFC is almost assured.
References
- Kerppola, T. K. Bimolecular fluorescence complementation: visualization of molecular interactions in living cells. Methods Cell Biol. 2008, 85, 431
- Vidi, P. A.; Przybyla, J. A.; Hu, C. D.; Watt, V. J. Visualization of G protein-coupled receptor (GPCR) interaction in living cells using bimolecular fluorescence complementation (BiFC). In Curr. Protoc. Neurosci. [Online]; Wiley and Sons: New York, 2010; Chapter 5, pp 1
- Milligan, G. A day in the life of a G protein-coupled receptor: the contribution to function of G proteincoupled receptor dimerization. Br. J. Pharmacol. 2008, 153, 216
- Maurice, P.; Kamal, M.; Jockers, R. Asymmetry of GPCR oligomers supports their functional relevance. Trends Pharmacol. Sci. 2011, 32, 514.
- Rozenfeld, R.; Devi, L. A. Receptor heteromerization and drug discovery. Trends Pharmacol. Sci. 2010, 31, 124
- Terrillon, S.; Bouvier, M. Roles of G-protein coupled receptor dimerization. EMBO Rep. 2004, 5, 30
- Kaczor, A. A.; Selent, J. Oligomerization of G protein-coupled receptor: biochemical and biophysical methods. Curr. Med. Chem. 2011, 18, 4606
- Vidi, P. A.; Ejendal, K. F.; Przybyla, J. A.; Watts, V. J. Fluorescent protein complementation assays: new tools to study G protein-coupled receptor oligomerization and GPCR-mediated signaling. Mol. Cell Endocrinol. 2011, 331, 185
- Kerppola, T. K. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat. Protoc. 2006, 1, 1278
- Vidi, P. A.; Chen, J.; Irudayaraj, J. M.; Watts, V. J. Adenosine A2A receptors assemble into higher-order oligomers at the plasma membrane. FEBS Lett. 2008, 582, 3985
- Vidi, P. A.; Chemel, B. R.; Hu, C. D.; Watts, V. J. Ligand-dependent oligomerization of dopamine D2 and adenosine A2A receptors in living neuronal cells. Mol. Pharmacol. 2008, 74, 544
- Przybyla J. A.; Watts, V. J. Ligand-induced regulation and localization of cannabinoid CB1 and dopamine D2L receptor heterodimers. J. Pharmcol. Exp. Ther. 2010, 332, 710
- Kearn, C. S.; Blake-Palmer, K.; Daniel, E.; Mackie, K.; Glass, M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptor enhances heterodimer formation: a mechanism for receptor cross-talk? Mol. Pharmacol. 2005, 67, 1697.
- Vidi, P. A.; Watts, V. J. Fluorescent and bioluminescent protein-fragment complementation assays in the study of G protein-coupled receptor oligomerization and signaling. Mol. Pharmacol. 2009, 75, 733
- Rebois, R. V.; Robitaille, M.; Petrin, D.; Zylbergold, P.; Trieu, P.; Hebert, T. E. Combining protein complementation assays with resonance energy transfer to detect multipartner protein complexes in living cells. Methods 2008, 45, 214
- Gandia, J.; Galino, J.; Amaral, O. B.; Soriano, A.; Lluis, C.; Franco, R.; Ciruela, F. Detection of higher-order G protein-coupled receptor oligomers by a combined BRET-BiFC technique. FEBS Lett. 2008, 582, 2979
- Filizola, M. Increasingly accurate dynamic molecular models of G-protein coupled receptor oligomers: Panacea or Pandora’s box for novel drug discovery? Life Sci. 2010, 86, 590
- Tadagaki, K.; Jockers, R.; Kamal, M. History and biological significance of GPCR heteromerization in the neuroendocrine system. Neuroendocrinology DOI:10.1159/000330000. Published online: Dec 9, 2011.
- Dalrymple, M. B.; Pfleger, K. D.; Eidne, K. A. G protein-coupled receptor dimers: functional consequences, disease states and drug targets. Pharmacol. Ther. 2008, 118, 359
- Goncalves, S. A.; Matos, J. E.; Outeiro, T. F. Zooming into protein oligomerization in neurodegeneration using BiFC. Trends Biochem. Sci. 2010, 35, 643
- Song, Y. H.; Wilmanns, M. Biomolecular fluorescence complementation in structural biology. Methods 2008, 45, 219
- Wang, R.; Li, Q.; Helfer, C. M.; Jiao, J.; You, J. The bromodomain protein Brd4 associated with acetylated chromatin is important for maintance of higher-order chromatin structure. J. Biol. Chem. DOI:10.1074/JBC.M111.323493. Published online: Feb 10, 2012.
- Hernandez, F. P.; Sandri-Goldin, R. M. Bimolecular fluorescence complementation analysis to reveal protein interactions in herpes virus infected cells. Methods 2011, 55, 182
- Kodama, Y.; Hu, C. D. An improved bimolecular fluorescence complementation assay with a high signal-tonoise ratio. Biotechniques 2010, 49, 793